Enviro Process Engineering (HK) Co. Ltd.
安和環保工程(香港)有限公司
The most common air disinfection method is using ultraviolet (UV) radiation.
UV radiation (UV-C) kills bacteria and viruses by damaging the DNA/RNA of the cells of microorganisms. However, UV radiation could only disinfect air close to the lamps as UV light has limited penetration capacity. In case of SARS contaminated room, UV disinfection alone is not adequate to provide virus-free environment for us.
Another well-known air cleaning method is to employ High Efficiency Particulate Air (HEPA) filter. HEPA filter can capture particulate sizes down to 0.3 microns, and so bacteria with size larger than 0.3 microns could be trapped in the filter.
Although HEPA filters are effective in reducing airborne bacteria in air, it is not effective to remove viruses, which are nanometer (10-9 m) in size. Also, air must pass through the filter in order for it to be cleaned. Hence HEPA filters can only clean air that is within a short distance of the HEPA unit. These drawbacks make HEPA filters become an unsatisfactory candidate for disinfection of SARS contaminated areas.
Chemical disinfectants could also be used for air disinfection, usually by means of vaporizing or spraying. However, these chemical disinfectants are usually difficult to decompose, leaving toxic chemical residues that are hazardous to human health.
Ozone is a well-known powerful oxidizer which could kill microorganisms effectively. Ozone applications in water and wastewater treatments are well-documented and it is widely used by most of the modern cities. Although studies for using ozone to disinfect air are relatively limited, experimental results (2,3) indicate that ozone could also be an effective air disinfectant as in water. For example, Kowalski et al (2) investigated the bactericidal effects of high ozone concentrations on E. coli and S. aureus and concluded that more than 99.99% death rate was achieved for both species after ozonation.
In addition to the strong oxidizing power of ozone, properties of ozone also help it to be an ideal aerial disinfectant. In contrast to UV radiation and HEPA filter, ozone is a gas that could penetrate to every corners of the room, thus it could disinfect the entire room effectively. As ozone is unstable, it is readily converted back to oxygen, leaving no harmful residual ozone after disinfection.
Although ozone is success as an aerial disinfectant in laboratory experiments (1), its effectiveness in real situation needs to be further explored. In this article, the effectiveness of ozone in disinfection of a conference room will be evaluated and discussed.
Ozone (O3) is an unstable gas comprising three atoms of oxygen. It is unstable because the gas will readily degrade back to its stable state, diatomic oxygen (O2) with the formation of free oxygen atoms or free radicals. The free oxygen atoms or radicals are highly reactive and they will oxidize almost anything (including viruses, bacteria, organic and inorganic compounds) in contacts, making ozone an enormously powerful disinfectant and oxidizer.
In fact, ozone is a much stronger oxidizer than other common disinfectants such as chlorine and hypochlorite. The usage of chlorine or hypochlorite in many countries has been decreased significantly due to the possibility formation of carcinogenic by-products such as trihalomethanes (THM) during the disinfection process. In contrast, ozone disinfection does not produce any harmful residues, and all the residual ozone will be converted back to oxygen within a short time. Ozone is therefore considered as an environmentally friendly disinfectant.
Given its superior strength and effectiveness as an oxidant and biocide, ozone becomes one of the dominant water treatment technologies in Europe and America. But its application in air disinfection is not as popular as water due to the concern on ozone’s toxicity. Ozone with concentration higher than 1 ppm has adverse effects on human health and the use of ozone for air disinfection is generally not recommended if people are around. Therefore, air disinfection using ozone should be restricted to unoccupied room only.
Procedure for air disinfection using ozone
In order to test for the effectiveness of ozone in reducing airborne bacteria, a conference room with area about 12m2 was selected for testing. As high level of ozone is required to kill viruses, bacteria and spores, the disinfection process was carried out when humans, animals and plants were evacuated.
Depends on the size of the room, an ozone generator with 2g/hr output was chosen. The capacity of the chosen ozone generator has the ability to maintain high concentration of ozone (0.5 – 5 ppm) inside. Circulation fan was placed in the room to ensure good distribution of ozone. After closing all the windows and doors, the ozone generator was turned on by remote device located outside to begin ozonation process. Concentration of ozone was monitored using a digital ozone sensor (Ecosensor). Different levels of ozone (0.5, 2.5 and 5 ppm) were tested to determine the optimal value for killing as much microorganisms as possible. After turning off the ozone generator, ozone level began to drop as it was undergoing self-decomposition to oxygen.
For safety reason, no people should enter the room until the level of residual ozone is below 0.02 ppm. In general, ozone concentration drops to below 0.02 ppm in a hour after ozonation, therefore people should wait for at least one hour (after turning off the generator) before entering the “ozonated” room.
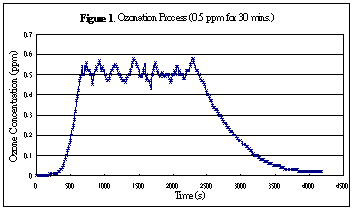
Figure 1 shows a typical curve (concentration vs. time) during ozonation process. As shown in the figure, the ozone concentration raise very slowly in the initial period (the first few minutes). The delay in building up the ozone concentration is probably due to the consumption of ozone for oxidizing pollutants (including bacteria and viruses) in the initial period. After oxidizing the major pollutants, ozone concentration inside the room raise rapidly up to the desired level. To ensure entire room disinfection, high level of ozone was maintained for 30 minutes. When ozone generator was off, the ozone concentration dropped gradually as ozone converting back to oxygen

The total airborne bacteria in the conference room was measured before and after each ozonation. Measurement was carried out using an Andersen N-6 single-stage sampler with Tryptone Soya Agar (Oxoid) in petri dish. 283L of air was taken for each sampling. The petri dish was incubated at 35oC for 48 hrs before counting. The disinfection efficiency of ozonation at different concentration was tabulated in Table 1.
|
Ozone conc. |
0.5 ppm |
2.5 ppm |
5 ppm |
|
Before Ozonation |
592 CFU/m3 |
612 CFU/m3 |
552 CFU/m3 |
|
After Ozonation |
169 CFU/m3 |
42 CFU/m3 |
57 CFU/m3 |
|
Reduction % |
71.5% |
93.1% |
89.6% |
The results show that ozone is effective in reducing airborne bacteria. At higher ozone level, the sanitizing effect increased. Over 90% of airborne bacteria could be reduced at 2.5 ppm concentration. Further increase of ozone concentration to 5 ppm does not beneficial in bacteria reduction percentage.
Unlike laboratory experiments conducted by Kowalski et al (1) that could remove 99.99% airborne bacteria after ozonation, the best reduction percentage in our case was around 93% only. High removal percentage could not be achieved because the conference room was not 100% sealed. Doors should be opened briefly during each air sampling (for placing a new agar dish on the sampler) and air exchange from outside was unavoidable.
For safety reason, excessive high concentration ozone should be avoided and the lowest ozone concentration that could kill most of the microorganisms should be selected as optimum. Depends on the contamination level, 0.5 – 2.5 ppm ozone level is adequate for air disinfection.
Experimental data shows that ozone is effective in reducing airborne bacteria of unoccupied room. Over 90% of airborne bacteria could be reduced after ozonation. As viruses are generally more susceptible to ozone than bacteria, it could assume that all viruses are killed if large percentage of airborne bacteria are removed. Ozone is a gas that has good penetration capacity and powerful oxidizing power, thus its disinfection efficiency is superior to UV radiation and HEPA filter. As ozone disinfection is conducted in unoccupied room only and all the residual ozone will be decomposed after the treatment, ozone toxicity to human is therefore not a concern. Given the advantages of strong oxidizing power, good penetration capacity and no harmful residues left after the treatment, ozone is also recommended to be used in disinfection of SARS-contaminated environments.
1. Gérard V. Sunnen, SARS and Ozone Therapy: Theoretical Considerations, http://www.triroc.com/sunnen/topics/sars.html (2003).
2. W. J. Kowalski, W. P. Bahnfleth, and T. S. Whittam, Ozone Sci. & Eng., 20, 205-221 (1998).
3. T. Masaoka; Y. Kubota, S. Namiuchi, T. Takubo, T. Ueda, H. Shibata, H. Nakamura, J. Yoshitake, T. Yamayoshi, H. Doi, T. Kamiki, Appl. & Environ. Microb., 43, 509-513 (1982).
Room 1019, Nan Fung Comm. Ctr., 264-298 Castle Peak Road, Tsuen Wan, N.T. Hong Kong
香港新界青山道264-298号南豐商業中心1019室
Tel 电話: (852) 2611-7376 Fax 傳真 : (852) 2617-5716 E-Mail电郵 : envirohk@netvigator.com